Stem cells hold incredible potential in regenerative medicine, offering promise for treating a wide range of diseases. However, turning this potential into reality is a challenging process that involves overcoming numerous technical hurdles, including the need for efficient, scalable, and cost-effective cell production. This is where automation can play a crucial role, enabling researchers to achieve consistent results while scaling up production for industrial and therapeutic applications.
Why Automation in Stem Cell Workflows?
Historically, stem cell research has relied heavily on manual techniques. This requires highly skilled labor and is prone to contamination, variability, and scalability issues. Manual cell culture can be highly time-consuming and labor-intensive, limiting the number of experiments that can be performed and restricting the throughput needed for large-scale research projects or clinical applications. For instance, in the study by Truong et al. (2021), an automated platform (TECAN Fluent) was used to culture and differentiate human induced pluripotent stem cells (hiPSCs) into retinal pigment epithelium (RPE). This automated approach enabled reproducible, and scalable generation of RPE cells, illustrating the potential for automation in personalized drug testing and disease modeling for age-related macular degeneration (AMD) patients. A similar approach can be implemented virtually on any other liquid handling robots such as Hamilton.
The Benefits of Automation in Stem Cell Culture
There are multiple advantages to integrate automation in cell culture workflow which I listed a few key ones with examples from recent publications below:
- Enhanced Efficiency and Throughput: Automated systems can perform routine tasks such as media changes, cell seeding, and harvesting at a faster pace and with higher precision than manual methods. For example, Bando et al. (2022) introduced a compact, automated culture machine designed for maintaining and differentiating hiPSCs. This system successfully expanded hiPSC cultures under feeder-free conditions and simultaneously differentiated them into cardiomyocytes, hepatocytes, neural progenitors, and keratinocytes. This machine uses a novel x-y-z-axes-rail-system to perform automated cell culture processes, making it easier for research laboratories to adopt iPSC-based workflows without the need for extensive space or specialized training. This level of automation reduces the need for highly trained personnel and supports high-throughput screening in various research fields.
- Improved Reproducibility and Consistency: One of the main advantages of automation is its ability to reduce variability and human error. This is particularly important in stem cell research, where even small variations in culture conditions can significantly impact cell behavior and differentiation outcomes. Automating these processes ensures that experiments are reproducible and results are consistent across different batches and laboratories.
- Reduced Contamination and Safety Risks: Automation minimizes human intervention, thereby reducing the risk of contamination and exposure to hazardous materials. This is especially important when working with stem cells for clinical applications, where sterility and safety are paramount.
- Scalability for Large-Scale Production: Automated systems can easily be scaled up to handle large numbers of cell cultures and experiments simultaneously. This capability is essential for translating stem cell research from the lab to the clinic and supporting commercial-scale production of cell therapies. Elanzew et al. (2020) reported the development of the StemCellFactory, a modular platform that covers the entire hiPSC production workflow, from reprogramming human fibroblasts to expanding hiPSC clones. The platform integrates advanced hardware and software components, enabling parallel processing of multiple hiPSC lines and providing a scalable solution for disease modeling and drug screening.
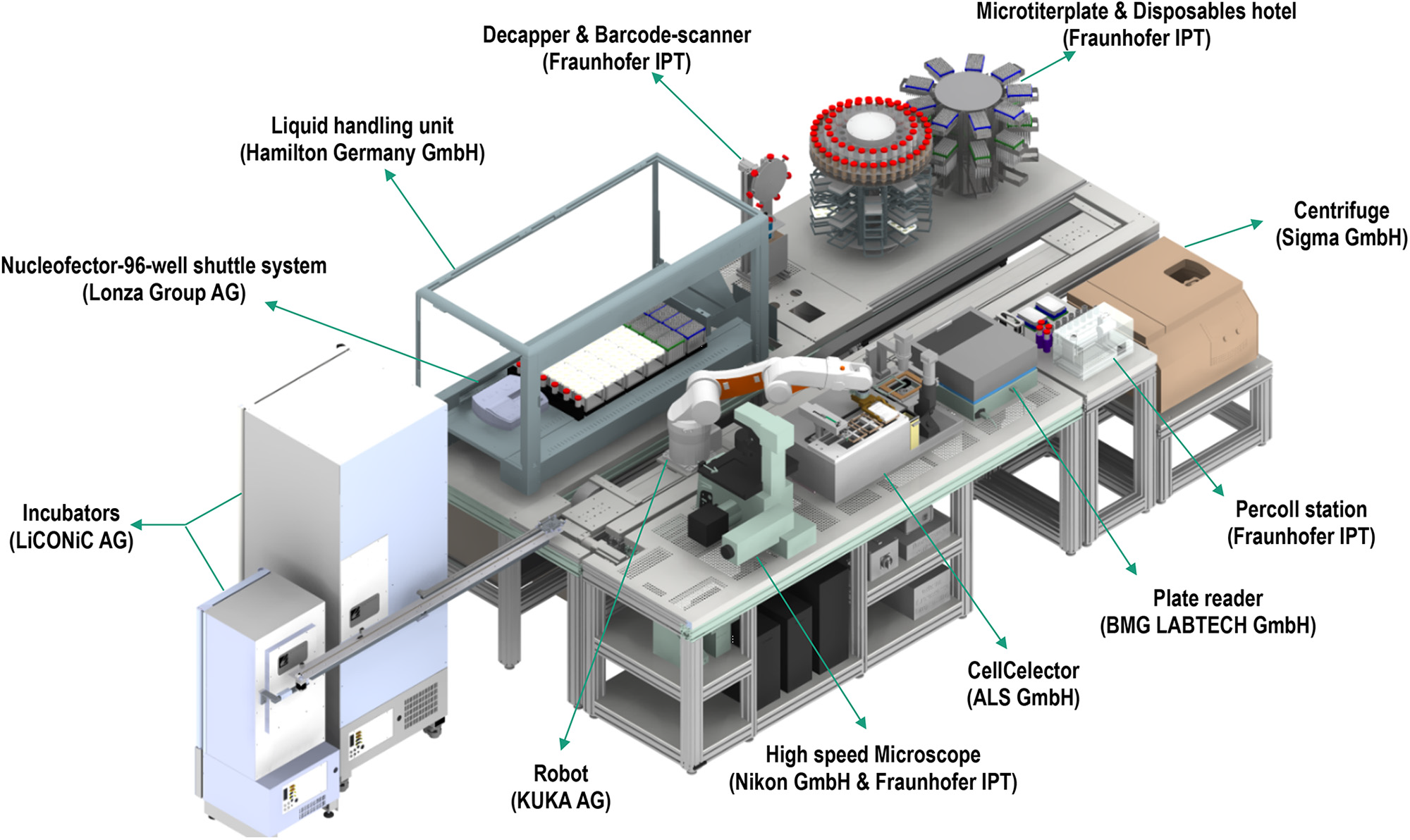
Figure 1. The StemCellFactory, an automated system for reprogramming and expansion of iPSCs. From DOI: 10.3389/fbioe.2020.00811
Market Trends, Future Directions, and Challenges
As the demand for stem cell therapies and personalized medicine grows, the market for automated cell culture systems is expected to expand rapidly. The global automated cell culture market was valued at USD 18.75 billion in 2023 and is projected to reach USD 47.50 billion by 2032, growing at a compound annual growth rate (CAGR) of 11.28%. This growth is driven by increasing adoption of automated technologies in both research and industrial settings. As automation platforms become more accessible, modular, and user-friendly, they will likely play a critical role in enabling the widespread adoption of stem cell technologies for clinical and commercial applications.
Despite the numerous benefits, there are still challenges to the widespread adoption of automation in stem cell research. Setting up automated systems can be expensive, especially for smaller research laboratories. In addition, operating and maintaining automated systems requires specialized knowledge and skills. Researchers may need additional training to fully utilize the capabilities of these systems. The other challenge is to integrate automation when the lab is already using manual techniques. However, the long-term benefits in terms of reduced labor costs and increased efficiency often justify the investment.
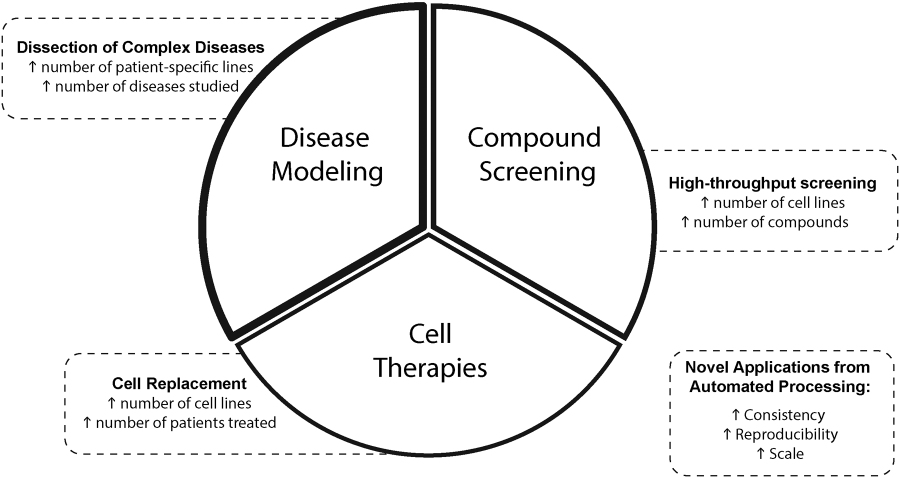
Figure 2. Schematic representation of novel or facilitated applications for hPSCs by automation. From DOI: 10.1177/247263031771222
Conclusion
The automation revolution in stem cell research is transforming the field by enabling high-throughput, reproducible, and scalable workflows. From compact, automated culture machines to large-scale platforms like the BioNex Solution’s Hive and CELLITRO RoboCell, automation is addressing the challenges of manual cell culture and making stem cell research more accessible and effective. As these technologies continue to evolve, they will play an increasingly important role in translating the promise of stem cells into real-world therapies and clinical applications.
If there is a topic that you would like to see here or have a question, please drop us a line at hello@assay.dev
