The U.S. Food and Drug Administration (FDA) is a cornerstone of public health, tasked with ensuring that medical treatments are both safe and effective. This vital agency balances its duty to protect consumers with the goal of expediting access to life-saving drugs and therapies. While the FDA is occasionally criticized for its lengthy and costly approval processes, these steps are crucial for maintaining safety and efficacy standards.
In this summary, I’ll briefly walk through the FDA’s drug approval process, the key phases of clinical trials, the types of FDA meetings, and their significance for researchers and sponsors.
What is the FDA Drug Approval Process?
A drug, as defined by the FDA, is any substance intended for diagnosing, treating, curing, mitigating, or preventing disease. It may also alter the structure or function of the body and is often a critical component of medical advancements.
Timeline of Drug Approval
The FDA drug approval process is rigorous, averaging 12 years and over $1 billion from concept to market. It includes:
- Preclinical Studies: Initial research conducted in labs and animal models.
- Clinical Trials: A multi-phase process testing the drug in humans.
- New Drug Application (NDA): The formal request to gain FDA approval for manufacturing and marketing.
Breaking Down Clinical Trial Phases
The clinical trial process is segmented into multiple phases, each addressing specific questions about the drug’s safety and efficacy:
Phase 0: Exploratory Trials
- Purpose: To test the drug in humans for the first time.
- Scale: Very small groups (10–15 participants).
- Key Steps: Use less than 1% of the dose that is expected to have a clinical effect. Requires an exploratory Investigational New Drug (IND) application.
Phase I: Safety Evaluation
- Purpose: Identify a safe dosage range and side effects.
- Scale: 20–80 healthy volunteers.
- Characteristics: Trials often begin with single-dose studies, progressing to multiple doses.
Phase II: Efficacy and Safety
- Purpose: Assess effectiveness while continuing to monitor safety.
- Scale: 100–300 participants with the targeted condition.
Phase III: Large-Scale Studies
- Purpose: Confirm effectiveness, monitor side effects, and compare the drug to existing treatments.
- Scale: 1,000–3,000 patients with the targeted medical condition.
Phase IV: Post-Approval Research
- Purpose: Conduct long-term monitoring after FDA approval to gather additional safety and effectiveness data.
Key Applications in the Approval Process
- Investigational New Drug (IND) Application
Before beginning human trials, a sponsor must file an IND with the FDA. This application provides:
- Drug composition and manufacturing details.
- Preclinical data to establish safety.
- A rationale for testing the drug in humans.
In emergencies, researchers can request an Emergency IND (EIND) to use an investigational drug without delay.
- New Drug Application (NDA) or Biologics License Application (BLA)
Upon completing Phase III trials, a sponsor submits an NDA or BLA to request FDA approval for the drug’s production and sale. The NDA includes:
- Clinical trial data and findings.
- Details about the drug’s manufacturing process and facilities.
- Labeling, risk evaluation, and safety monitoring plans.
The FDA typically reviews NDAs within 10 months. Some drugs may qualify for accelerated review, expediting the timeline to 6 months for therapies addressing unmet medical needs.
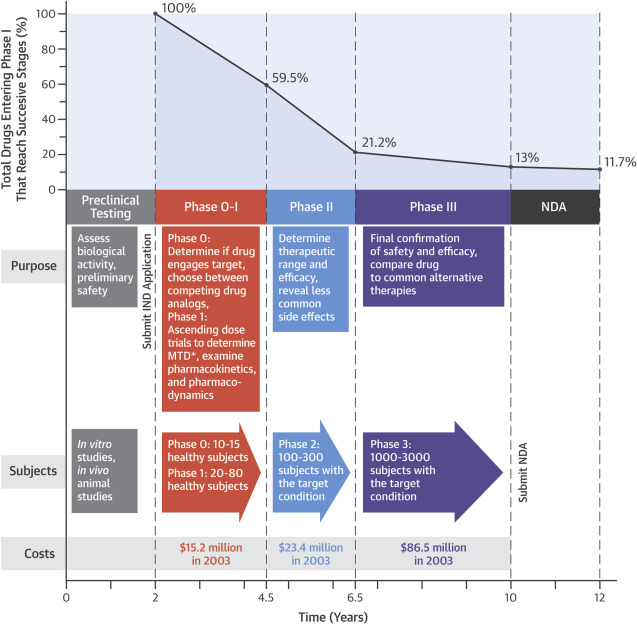
Figure 1 Time, Money, and Success: Stages in Drug Development . From here https://doi.org/10.1016/j.jacbts.2016.03.002
Understanding FDA Meetings
Formal FDA meetings play a pivotal role in drug development, offering sponsors guidance and feedback. These meetings are categorized based on purpose and urgency:
Type A Meetings
- Purpose: Resolve issues stalling drug development, such as clinical holds or disputes.
- Timeline: The FDA will respond to a request for a Type A meeting within 14 calendar days, and the meeting will be scheduled within 30 days of the request.
Type B Meetings
- Purpose: Discuss general drug development plans or review data at key milestones, such as end-of-phase reviews.
- Timeline: The FDA will respond to a request for a Type B meeting within 21 calendar days, and the meeting will be scheduled within 60 days of the request.
Type C Meetings
- Purpose: Address any topics outside Type A or B categories, often broader in scope. For example, if the sponsor wants to discuss the development and feasibility of a new biomarker or surrogate endpoint
- Timeline: The FDA will respond to a request for a Type C meeting within 21 calendar days, and the meeting will be scheduled within 75 days of the request.
Type D Meetings
- Purpose: Discuss focused, innovative development questions, typically limited to two key topics.
- Timeline: The FDA will respond to a request for a Type D meeting within 14 calendar days, and the meeting will be scheduled within 50 days of the request.
INTERACT (Initial Targeted Engagement for Regulatory Advice on CBER Products) Meetings
- Purpose: Early-stage discussions for innovative product development. For example, if the sponsor wants to propose innovative manufacturing processes for stem cell therapies or seek feedback on the use of a novel viral vector platform for gene delivery.
- Response Time: FDA The FDA will respond to a request for an INTERACT meeting within 21 days.
Requesting FDA Meetings
Sponsors must submit a written meeting request and package that includes:
- Product details (e.g., name, application number, and proposed indication).
- The purpose of the meeting.
- Proposed questions organized by discipline.
- Background on completed or planned studies.
Meeting packages must be submitted electronically and include a table of contents and additional context for reviewers. If approved, the FDA sends a letter confirming the meeting details, including date, time, and location.
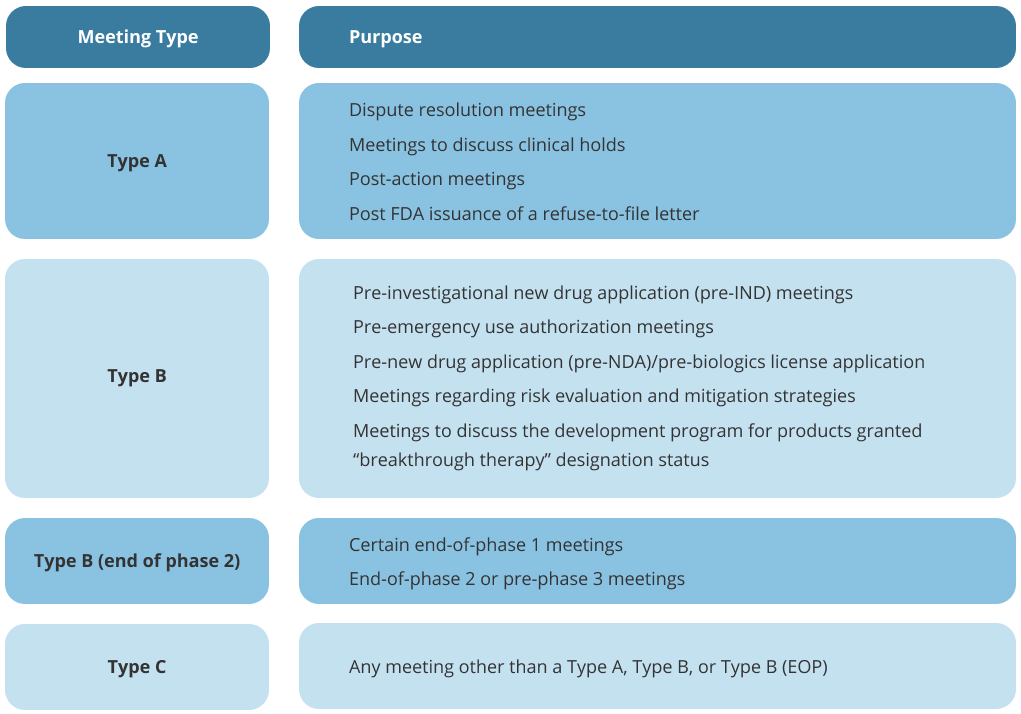
Figure 2. Summary of FDA meeting types. From here https://omarconsultants.com/services/strategic-clinical-design-and-regulatory-meetings/
Meeting Conduct and Documentation
During meetings:
- The FDA assigns a chairperson and reviews the agenda.
- No audio or visual recordings are allowed.
- Sponsors should present clear, focused questions (no more than 10 per meeting).
- Either party summarizes key discussion points and action items.
The FDA’s official meeting minutes serve as the formal record of discussions, agreements, and next steps.
Advisory Committee (AdComm) Meetings
Advisory committees provide expert recommendations on new medical products, focusing on safety, effectiveness, and benefit-risk assessments. These panels, composed of independent experts, offer input that helps the FDA make informed decisions.
Common Delays in FDA Meetings
Delays can occur due to:
- Voluminous or incomplete meeting packages.
- Late submission of additional questions or data.
- Scheduling conflicts with essential attendees.
Sponsors may also choose to cancel meetings if preliminary FDA responses address their concerns adequately.
Accelerating Drug Development
The FDA recognizes the need to expedite drug approvals without compromising safety. Initiatives like accelerated reviews for generic drugs and breakthrough therapies aim to streamline the process, ensuring that patients receive critical treatments faster.
Conclusion
The FDA’s drug approval process and formal meetings ensure a balanced approach to public safety and innovation. While the process can be complex and time-intensive, understanding the structure of clinical trials, key applications like INDs and NDAs, and the different types of meetings can empower sponsors to navigate the system more effectively. With its commitment to efficiency and safety, the FDA continues to be a vital partner in bringing transformative medical treatments to the public.
If there is a topic that you would like to see here or have a question, please drop us a line at hello@assay.dev
